Building Immunity from the Bottom Up
The Emmy Noether Research Group Immuno Materials develops new synthetic cell-based approaches to regulate and study immune reactions. By applying model membrane systems and in vitro reconstitution approaches, inspired from bottom-up synthetic biology, we engineer controlled cellular environments that trigger immune reaction in T cells and cancer cells. With this approach, we study how biophysical and biochemical cues guide cancer-immune signaling and apply this knowledge to improve next generation immunotherapies.

Mitarbeiter/innen
Research
Synthetic tumor immune microenvironments
Major research questions
How do cancer cells adapt to their immune microenvironment? How does the presence of immune cells around cancer cells affect their response to therapy? How do the biophysical properties of tumors change when immune cell infiltrate into them?
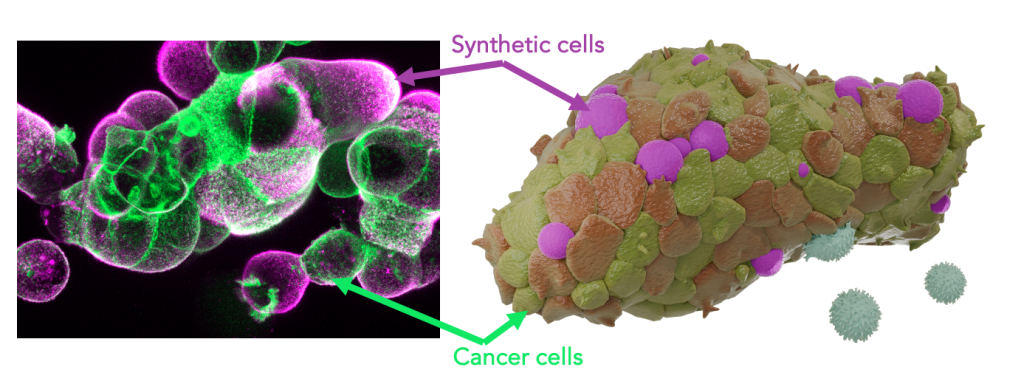
Within the framework of this project, we develop artificial tumour immune microenvironments (TIMEs) for human pancreatic cancer organoids empowering structured and rational analysis of tumour immune adaptations Immune cells, the defining elements of an immune microenvironment are recreated as synthetic cells by bottom-up assembly from their single molecular building blocks The programmable synthetic cells are introduced into tumour organoids to function as lifelike leukocyte mimics presenting immune effector functions By this, a molecularly defined immune environment is created inside tumour models Multi-parametric screenings assess organoid development as well as immunotherapy response as a function of the synthetic microenvironment configuration This strategy links TIME architectures to cancer immune adaptation and evasion for quantitative description of therapy resistance The project strives to de-convolute the dynamic complexity of the tumour immune microenvironment towards a rational dissection Moreover, it contributes concepts for the assembly of hybrid biomaterials that embody essential features of living cells.
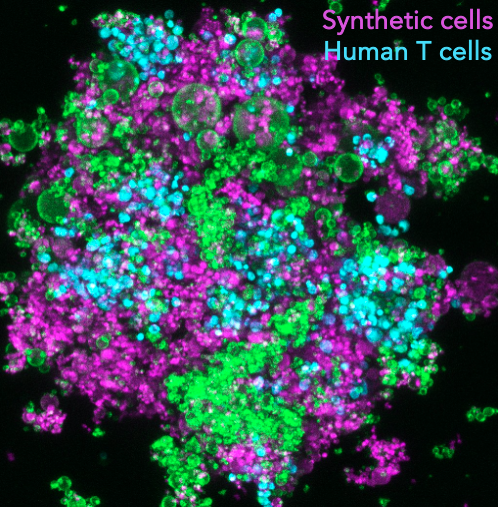
Synthetic lymphatic tissues
Major research questions
What are the microanatomical architectures and mechanical properties that favor T-cell stimulation in lymph nodes? What is the cross-correlation between mechanical and biochemical cues during T-cell activation? How do T cells collect and integrate signals within lymph nodes? How can T-cells be activated in a programmable, long-lasting and controlled way for therapeutic purposes?
T cells are instrumental in combatting diseases and fostering lasting immunity. Their maturation is a meticulously orchestrated process that takes place within lymph nodes, the central regulatory hubs for immunity. These nodes not only determine the stimulation of specific T cell types but also provide the optimal environment for their activation, ensuring a targeted and potent response. This maturation involves a sequence of events, including initial stimulation, monoclonal expansion, and differentiation. The sequence’s order and duration are vital for cultivating a diverse T cell population and a successful adaptive immune response. As T cells mature, the cellular microenvironment within the lymph nodes continually evolves, leading to changes in the biochemical and biomechanical properties of adjacent cells. Such alterations, like shifts in tissue stiffness or the introduction of various stimulatory ligands, guide T cell maturation and support their differentiation. For example, during T cell expansion, the lymph node environment sees about a 3-fold increase in stiffness, and nearby cells like antigen-presenting cells (APCs) or fibroblastic reticular cells (FRCs) release more stimulatory ligands and cytokines, such as IL-7 and CCL21. However, current in vitro T-cell expansion technologies fall short in replicating this intricate sequence of events. Our project aims to establish ex vivo T-cell expansion pipelines that mirror these in vivo processes, incorporating modules that adjust T-cell microenvironments using synthetic cells. This approach aims to boost the production of high-quality, therapeutic T cells for immunotherapy.
Synthetic tumor niches
Major research questions
How can leukemia cells hide in lymph nodes? Which survival and resistance signals do they receive there? How can we design ex vivo systems to mimic and study the relevant processes?
Leukemia, a form of blood cancer, remains a formidable challenge in oncology. Despite many patients initially responding to treatments and the advent of advanced immunotherapies that have notably enhanced prognoses, relapses remain a significant concern. This is largely attributed to certain leukemia cells, particularly cancer stem cells, finding sanctuary within lymph nodes. These nodes, under normal physiological conditions, offer a nourishing environment for white blood cells to proliferate and thrive. However, under pathological conditions, such as in cancer, they can inadvertently provide a protective haven for therapy-resistant leukemia cells. In this project, we aim to decipher the key signals lymph nodes extend to resident leukemia cells that confer therapy resistance. To achieve this, we build our own synthetic lymph nodes from the bottom-up, controlling their biochemical and biophysical properties aligned with those observed in leukemia patients. Subsequent treatments with chemotherapy and antibody immunotherapies allow us to evaluate how the lymph node environment potentially aids cancer cell survival. This endeavor, while rooted in fundamental science, holds promise for offering predictive insights in a translational context.
Research Philosophy
In the ever-evolving landscape of scientific research, our lab stands firm on certain foundational beliefs that guide our inquiries and innovations. These principles not only shape our approach but also define our commitment to advancing the frontiers of knowledge.
- Balancing Fundamental Science with Technological Advances: At the heart of our work is a deep respect for fundamental science, understanding its role as the catalyst for technological innovations. Conversely, we recognize that new technologies can illuminate and deepen our fundamental insights. In this symbiotic relationship, our lab harnesses both the foundational and the innovative aspects of the life sciences.
- Synthetic Biology & Understanding by Design: Merely observing and perturbing molecules, cells, and organisms isn’t enough to truly grasp the intricacies of biological systems. We embrace the principles of synthetic biology, advocating for an „understand-by-design“ approach. By adopting an engineering lens towards life, we seek to derive quantitative insights and fortify our scientific discourse with new empirical evidence.
- Insight Over Data: While data-driven research forms the bedrock of science, we believe that conceptual insights often hold greater value. We believe in the power of ideas and emphasizing the significance of arguments is rooted in experiments and mechanisms over mere data accumulation.
Beyond these foundational pillars, our lab emphasizes inclusivity and collaboration. We foster an environment where individuals, regardless of their career stage, are encouraged to share ideas and constructive critiques. Open dialogue is our strength; it’s through candid and sometimes challenging discussions that we unearth groundbreaking insights and concepts. Our ultimate goal is to cultivate a nurturing space where students flourish and scientists are equipped with all they need to discover new perspectives.
Success stories
Though our lab was only recently established, we’ve been at the forefront of pioneering new concepts in synthetic biology and immunotherapy. Our contributions have already made significant impacts.
Beyond the Spike: Pioneering Discoveries in the Heart of a Pandemic
The COVID-19 pandemic tested us all, both personally and professionally. As nations grappled with lockdowns, scientists globally ramped up their efforts to decode the mysteries of the SARS-CoV-2 virus. We all played our part.
In the summer of 2020, at the pandemic’s onset, we collaborated with an interdisciplinary team from the University of Bristol and the Max Planck Center Bristol in the UK. Our colleagues there achieved a groundbreaking discovery: they identified the molecular structure of the SARS-CoV-2 spike protein binding unexpectedly to a fatty acid, specifically linoleic acid (Toelzer et al. (2020) Science). This finding was monumental. The fatty acid essentially „locked“ the spike protein, preventing it from infecting cells. We soon recognized the profound implications this could have on our understanding of COVID-19.
As the pandemic evolved, we delved deeper into the fatty acid binding pocket’s role. Notably, all emerging SARS-CoV-2 variants retained this binding pocket. Using our understand-by-design approach, in partnership with the Max Planck Center, we crafted non-infective, molecularly defined minimal synthetic variants of SARS-CoV-2 virions. This allowed us to methodically analyze the fatty acid’s role in virus-cell docking (Staufer et al. (2021) Nature Communications). Our research led to a model that elucidated the evolutionary advantage of the fatty acid binding pocket: it shields the virus from immune detection. This molecular switch modulates the virus’s infectivity, adapting to the immune system’s local activity and inflammation.
Our minimal virions also shed light on the evolution and diversity of SARS-CoV-2 (Gupta et al. (2021) Nature Communications) and provided insights into how the virus impedes the formation of immune synapses (Onnis et al. (2022) Journal of Experimental Medicine). Our discoveries surrounding the fatty acid binding pocket even spurred the creation of Halo Therapeutics, a UK-based company now developing pan-coronavirus treatments. Halo just completed its Series A funding!
In summary, our journey has been both challenging and rewarding. We’ve made groundbreaking scientific discoveries, engaged in enriching discussions, and forged new partnerships, all underpinned by our understand-by-design approach. It’s a testament to the power of collaboration and innovation in the face of adversity.
Synthetic Surge: Engineering Life’s Blueprint in the New Age of Biology
In the realm of biology, the understand-by-design approach isn’t a newcomer. For decades, scientists have endeavored to recreate cellular life. Yet, it’s the emergence of advanced molecular systems engineering and the capability to craft supramolecular systems in test tubes that has truly transformed our pace. Now, we can swiftly replicate natural cells with varied functions, from cell division to regulating cellular immunity.
Armed with these cutting-edge tools, the global race towards synthetic cells is in full swing. Numerous labs and initiatives are sprouting, all with a shared vision: to birth the first living synthetic cells. While some delve deep into life’s fundamentals, others see a future where these synthetic cells, being more controllable, can revolutionize sustainability and medicine.
Spurred by the SynCell conference in 2021 and in collaboration with global partners, we’ve embarked on a mission to streamline these worldwide endeavors. Joining forces with early-career scientists from nations like the US, UK, Netherlands, Spain, Germany, France, Japan, and Australia, we’ve brainstormed ways to attract the next generation of innovators to this field. Our aspiration goes beyond just cells; we aim to build a community to engineer synthetic cells and organelles from the bottom-up! We’ve strategized to amplify the socioeconomic and technological impacts of synthetic cells and emphasized the importance of an interconnected, interdisciplinary research community (Staufer et al. (2021) eLife). By bolstering communication, sharing resources, and initiating educational programs, we believe in a sustainable future for this field.
Our journey, bridging continents and disciplines, stands as a testament to the collaborative spirit of science. Witnessing the passion and drive of early-career scientists has been turely enriching!
Open position
We pride ourselves on being a diverse team, both culturally and scientifically. Our expertise spans a wide range of the natural sciences, including biophysics, immunology, advanced microscopy, microfluidics, nanotechnology, drug delivery, immunotherapy, virology, molecular biology, membranes, electrophysiology, cell biology, physical chemistry, and more. We invite you to reach out and become a part of our vibrant community.
Postdoctoral Researchers: We have fully-funded postdoc positions available. If you’re considering fellowships, we provide guidance and support for applications, including but not limited to the Marie Curie Fellowship, DFG Walter Benjamin Program, and the Feodor Lynen Fellowship by the Humboldt Foundation. We value your unique research ideas for other programs and offer constructive discussions within our group to help you craft a successful proposal. For postdoc opportunities, please get in touch by sending your interest statement to us directly.
PhD Candidates: We invite applications for our fully funded PhD positions. Our research projects are co-defined with students, emphasizing interdisciplinary training and state-of-the-art methods spanning biophysics, microfluidics, immunology, advanced microscopy, and 3D cell culturing. Send us your motivation letter and CV. We’re eager to hear about your research ideas and welcome you to explore them in our lab.
Master’s Students: We offer opportunities for master’s thesis projects in areas like synthetic cells, immunotherapy, organoids, and biomaterials. While these subjects have a predefined framework, we encourage students to add their unique perspective. Every master’s student is mentored directly by an experienced PhD candidate or postdoc.
High School Students: Unsure about your academic path? Curious about the dynamics of a research lab? We offer short-term research internships, providing a glimpse into the world of natural sciences. Join us and get a hands-on experience.
Publikationen
Hakami, Niki | Burgstaller, Anna | Gao, Ning | Rutz, Angela | Mann, Stephen | Staufer, Oskar
Advanced Healthcare Materials , 2024, 13 (22), 2303334.
https://onlinelibrary.wiley.com/doi/full/10.1002/adhm.202303334
Advanced NanoBiomed Research , 2024, 4 (9), 2400037.
https://onlinelibrary.wiley.com/doi/full/10.1002/anbr.202400037
Burgstaller, Anna | Piernitzki, Nils | Küchler, Nadja | Koch, Marcus | Kister, Thomas | Eichler, Hermann | Kraus, Tobias | Schwarz, Eva C. | Dustin, Michael | Lautenschlaeger, Franziska | Staufer, Oskar
Small , 2024, 20 (37), 2401844.
https://onlinelibrary.wiley.com/doi/10.1002/smll.202401844
Jahnke, Kevin | Staufer, Oskar
Journal of extracellular vesicles , 2024, 13 (4), e12436.
https://doi.org/10.1002/jev2.12436
Piernitzki, Nils | Staufer, Oskar
Advanced Therapeutics , 2024, 7 (3), 2300340.
https://onlinelibrary.wiley.com/doi/10.1002/adtp.202300340
Buzas, Dora | Bunzel, Adrian | Staufer, Oskar | Milodowski, Emily J. | Edmunds, Grace L. | Bufton, Joshua | Vidana Mateo, Beatrice V. | Yadav, Sathish K.N. | Gupta, Kapil | Fletcher, Charlotte | Williamson, Maia K. | Harrison, Alexandra | Borucu, Ufuk | Capin, Julien | Francis, Ore | Balchin, Georgia | Hall, Sophie | Vega, Mirella V. | Durbesson, Fabien | Lingappa, Srikanth | Vincentelli, Renaud | Roe, Joe | Wooldridge, Linda | Burt, Rachel | Anderson, Ross J. L. | Mulholland, Adrian | Bristol UNCOVER Group | Hare, Jonathan | Bailey, Mick | Davidson, Andrew D. | Finn, Adam | Morgan, David | Mann, Jamie | Spatz, Joachim | Garzoni, Frederic
Antibody therapeutics , 2023, 6 (4), 277-297.
https://academic.oup.com/abt/advance-article/doi/10.1093/abt/tbad024/7320073

Cortacero, Kévin | McKenzie, Brienne | Müller, Sabina | Khazen, Roxana | Lafouresse, Fanny | Corsaut, Gaelle | Van Acker, Nathalie | Frenois, Francois-Xavier | Lamant, Laurence | Meyer, Nicolas | Vergier, Béatrice | Wilson, Dennis G. | Luga, Hervé | Staufer, Oskar | Dustin, Michael L. | Valitutti, Salvatore | Cussat-Blanc, Sylvain
Nature Communications , 2023, 14 7112.
https://www.nature.com/articles/s41467-023-42664-x
Nature nanotechnology , 2023, 19 3-4.
https://www.nature.com/articles/s41565-023-01509-w